Utility of BRAF V600E Mutation-Specific Immunohistochemistry in Detecting BRAF V600E-Mutated Gastrointestinal Stromal Tumors
Abstract
As patients with BRAF V600E mutation respond to BRAF inhibitors, it is important to identify these mutations to stratify patients for the appropriate therapy. In this study, we evaluated the utility of a BRAF V600E allele-specific antibody in gastrointestinal stromal tumors (GISTs).
BRAF V600E mutation-specific immunohistochemistry (negative, weak, or moderate/strong expression) and BRAF sequencing were performed on 38 consecutive GISTs diagnosed between January 2013 and April 2014.
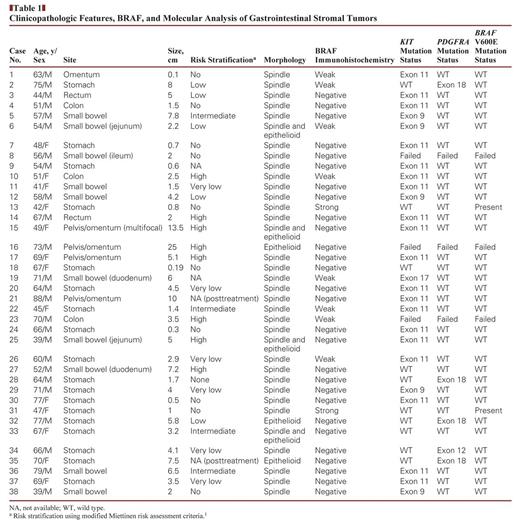
GISTs from a cohort of 25 men and 13 women (mean age, 61 years; range, 39–88 years) were localized to the stomach (18), small bowel (10), colon (three), rectum (two), and pelvis/omentum (five). Strong and diffuse cytoplasmic BRAF expression was noted in two (5%) of 38 cases, while eight (21%) of 38 cases showed weak staining, and 28 (74%) of 38 cases were negative. Both of the strongly positive cases arose in the stomach, occurring in a 42-year-old and a 47-year-old woman, respectively. The lesions measured 0.8 and 1 cm, showed spindle cell morphology, and had no risk of progressive disease by Miettinen criteria. Both cases showed heterozygous BRAF V600E, while no BRAF mutations were detected in cases with weak or negative BRAF expression.
BRAF V600E mutation-specific immunohistochemistry is a highly sensitive and specific method for detecting BRAF-mutated GISTs.
Clinicopathologic Features, BRAF, and Molecular Analysis of Gastrointestinal Stromal Tumors

Open in new tab
Materials and Methods
Gastrointestinal stromal tumor (GIST) is the most common mesenchymal tumor of the gastrointestinal tract. It predominantly arises in the stomach and small bowel and demonstrates a spindled and/or epithelioid morphology. Nearly 85% of GISTs harbor oncogenic mutations in KIT or PDGFRA.1 The most frequent KIT mutations occur in exon 11 (65%–70%) and exon 9 (20%), while exon 18 (6%) is the most common site for PDGFRA mutations.2–4 The remaining 10% to 15% of GISTs that do not harbor KIT or PDGFRA mutation are designated as wild-type GISTs. Recent studies have shown that the subset of KIT/PDGFRA wild-type GISTs is actually a heterogeneous group of tumors that often do not respond to tyrosine kinase inhibitors and harbor alterations in the succinate dehydrogenase (SDH) complex (SDH-deficient GISTs),5–8BRAF,9 or NF1.10 Identification of these alternative pathways of oncogenesis has not only provided an explanation for drug resistance but has also yielded new opportunities for targeted therapy, especially the use of BRAF inhibitors for BRAF-mutated GISTs.11
The most common, activating BRAF mutation occurs in exon 15 and is a DNA base substitution of thymine for adenine (T to A) that converts valine to glutamic acid of amino acid residue 600 (BRAF V600E). A variety of molecular assays, such as Sanger sequencing, pyrosequencing, allele-specific real-time polymerase chain reaction (PCR), mass spectrometry–based sequencing, and high-resolution melting curve analysis, have been used to detect BRAF V600E mutation.12 More recently, a BRAF V600E mutant-specific monoclonal antibody (clone VE1) was introduced and used to detect the BRAF-mutant protein in formalin-fixed, paraffin-embedded tissue obtained from various malignancies, including melanoma, thyroid cancer, and colorectal adenocarcinomas.13–18 However, the utility of this BRAF V600E mutant-specific monoclonal antibody in the detection of BRAF-mutated GISTs has never been evaluated and forms the basis of this study.
Following approval by Cleveland Clinic’s Institutional Review Board, consecutive GISTs diagnosed between January 2013 and April 2014 were culled from our database. In addition to documenting demographic data, we recorded the following parameters: location, size, primary/recurrent lesion, cell type (spindle, epithelioid, or mixed), mitotic activity, and immunoreactivity for CD117, CD34, and/or DOG1. Risk stratification for progressive disease was performed using the Miettinen risk assessment system.1
Immunohistochemical staining was performed on formalin-fixed, paraffin-embedded tissue cut in 4-μm sections on the Ventana Benchmark Ultra automated immunostainer (Ventana Medical Systems [VMS], Tucson, AZ). Online deparaffinization was followed by online epitope retrieval using a high pH Tris-based solution (Ultra CC1; VMS) for 64 minutes at 100°C. The slides were incubated with the anti-BRAF V600E primary antibody (mouse monoclonal VE1; Spring Bioscience, Pleasanton, CA) at 1:175 dilution for 16 minutes at 37°C. Localization of the antigen-antibody complex was achieved using the VMS OptiView DAB detection kit and VMS OptiView Amplification kit. Staining was scored as negative (no staining), weak (pale brown staining that was stronger than background staining in smooth muscle), or moderate/strong (intermediate to dark brown staining). Two authors (D.T.P. and B.P.R.) scored the slides, with discrepancies being resolved across the microscope.
Results
Histologic Review
DNA extraction was performed on formalin-fixed, paraffin-embedded tumor samples by macrodissecting tumor-rich areas from unstained paraffin sections. Selected exons of BRAF,15KIT,9,11,13,17 and PDGFRA12,14,18 were amplified by PCR and screened for sequence alterations by high-resolution melting curve analysis on an LC480 Lightcycler (Roche Applied Science, Penzberg, Germany). Primer sequences and PCR conditions are detailed in Data Supplement 1 (all supplemental materials can be found at http://bit.ly/PatilNov15). All mutations were confirmed by bidirectional Sanger sequencing as previously described.19,20 All immunohistochemical analysis and genotyping were performed in a blinded manner.
Immunohistochemical Analysis
The cohort consisted of 38 GISTs (37 primary and one recurrent) resected from 25 men and 13 women. The mean age was 61 years (range, 39–88 years). The tumors were localized to the stomach (18 [47%]), small bowel (10 [26%]), colon (three [8%]), rectum (two [5%]), and omentum/pelvis (five [13%]). Of the 38 cases, 35 were unifocal and three were multifocal. The mean size was 4.3 cm (range, 0.1–25 cm). Most tumors showed spindled morphology (31 [82%]), followed by mixed epithelioid and spindle (four [11%]), and epithelioid (three [8%]) phenotype Table 1. All tumors were positive for CD117 (KIT) and DOG1.
Strong and diffuse cytoplasmic BRAF expression was observed in two (5%) of 38 cases, which were also CD117 (KIT) and DOG1 positive Image 1. Both the cases were localized to the stomach and were found incidentally in a 42-year-old and a 47-year-old woman who underwent sleeve gastrectomies for morbid obesity. The lesions measured 0.8 cm and 1 cm in the greatest dimension, displayed spindle cell morphology, and showed no risk of progressive disease as assessed by modified Miettinen risk assessment criteria (Image 1). No histologic features distinguished these tumors from the more common KIT-mutated tumors. Both patients are alive without evidence of disease after a follow-up duration of 7 months and 3 months.
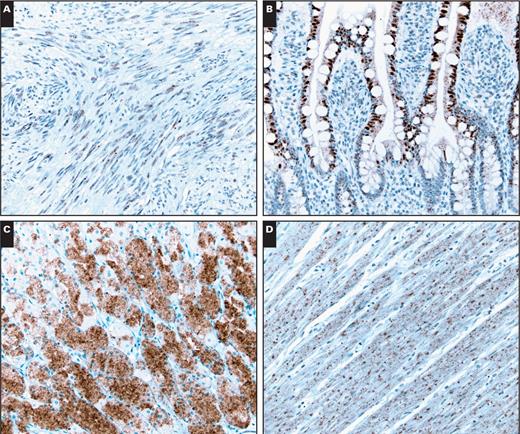
A, An example of a gastrointestinal stromal tumor with weak BRAF expression (×200). This case was negative for BRAF V600E mutation. Nonspecific staining was observed within the nuclei of small bowel epithelial cells (B, ×200), cytoplasm of parietal cells (C, ×200), and smooth muscle cells (D, ×200).
A, An example of a gastrointestinal stromal tumor with weak BRAF expression (×200). This case was negative for BRAF V600E mutation. Nonspecific staining was observed within the nuclei of small bowel epithelial cells (B, ×200), cytoplasm of parietal cells (C, ×200), and smooth muscle cells (D, ×200).
Genotyping
Discussion
Clinicopathologic Findings
Gain-of-function BRAF mutations have been detected in several malignancies, including colonic adenocarcinoma,24–26 papillary thyroid carcinoma,18 and melanoma,12 among others. Historically, these mutations have been detected using sequencing-based techniques. More recently, the BRAF V600E mutant-specific antibody VE1 became available for detecting BRAF mutations via immunohistochemistry.27 Studies that have evaluated this antibody in papillary thyroid carcinoma,28 melanoma,29,30 and pulmonary adenocarcinoma31 have demonstrated high sensitivity and specificity in detecting BRAF mutations. However, false-positive17,32,33 and false-negative results16 in colonic adenocarcinomas have questioned their utility as a primary tool for establishing BRAF mutation status. As the utility of the VE1 antibody in detecting BRAF mutations in GISTs has never been evaluated, we decided to perform this systematic analysis on a series of consecutive GISTs diagnosed at the Cleveland Clinic.
In our series, strong and diffuse BRAF expression was observed in two (5.3%) of 38 cases. Sequencing analysis confirmed the presence of BRAF V600E mutation in both cases, thus documenting 100% specificity and 100% sensitivity in detecting BRAF mutation using the VE1 antibody. Both these tumors were incidentally found within the stomach and measured 0.8 cm and 1.0 cm in the greatest dimension. Based on prior studies that have examined BRAF mutations in GISTs (summarized in Table 2), most BRAF-mutated tumors appear to originate within the small bowel and demonstrate spindle cell morphology. The stomach is an uncommon site of origin, and only four examples of gastric BRAF-mutated GISTs have been described thus far.9,34–36 Similar to our cases, all four cases of gastric BRAF-mutated GISTs reported in the literature demonstrated spindle cell morphology (Table 2). The tumor sizes (documented in three cases) and risk of progressive disease were 3 cm (very low), 4 cm (very low), and 10.5 cm (intermediate). Similar to prior studies, we did not find any histologic features that were distinctive of BRAF-mutated GISTs.
The reported frequency of BRAF mutations among wild-type GISTs has ranged from three (5%) of 6136 to two (7%) of 28,9 nine (13%) of 70,35 and three (20%) of 15.34 In our series, a BRAF mutation was found in two (40%) of five KIT/PDGFRA wild-type GISTs. This higher rate is most likely a result of the smaller sample size and the type II error introduced therein. Nonetheless, determining the rate of BRAF mutations in wild-type GISTs was not the goal of this study.
With one exception,37 current and previous studies9,35,36 have shown BRAF and KIT/PDGFRA mutations to be mutually exclusive genetic events in treatment-naive GISTs. Miranda et al37 reported a BRAF mutation in a GIST with a concomitant KIT exon 11 deletion (ΔV555-K558). This tumor was located within the small bowel and was risk stratified to have a high risk of progressive disease. In vitro experiments performed by Miranda et al showed that in cell lines coexpressing an imatinib-sensitive mutant form of KIT and constitutively activated BRAF protein, imatinib was able to inhibit KIT and its downstream signaling but not the extracellular signal–regulated kinase 1/2 activation driven by mutationally activated BRAF. This suggests that mutant BRAF causes resistance to imatinib and furthermore that KIT/PDGFRA wild-type tumors with BRAF mutations might be unresponsive to KIT inhibitors. A case reported by Falchook and colleagues11 supports this view. A 60-year-old man with BRAF V600E GIST had disease recurrence while on adjuvant imatinib and again while on adjuvant sunitinib following a second surgery. Importantly, this patient’s tumor did respond well to the BRAF inhibitor dabrafenib.11 Given that there are now three therapies approved by the US Food and Drug Administration targeting BRAF V600E (vemurafenib, dabrafenib, and trametinib), screening for this mutation in KIT/PDGFRA wild-type GISTs is clinically important.
Nonspecific staining within the epithelial cells (nuclear in small bowel epithelial cells and cytoplasmic in parietal cells) and smooth muscle cells was observed in a subset of our cases (Image 2). This phenomenon has been previously reported and is believed to be a result of cross-reactivity with other epitopes present in normal tissues.17,33,38,39
In summary, in addition to expanding the clinicopathologic profile of gastric BRAF-mutated GISTs, this study demonstrates the high sensitivity and specificity of VE1 antibody in detecting BRAF-mutated GISTs. This technique serves as an efficient tool to stratify patients with KIT/PDGFRA wild-type GISTs. We recommend using the VE1 antibody as a reflex test in routine practice, specifically in unresectable/metastatic GISTs that are negative for KIT/PDGFRA mutation and that would benefit from medical therapy.
Comparison of BRAF Immunohistochemistry and Molecular Genetic Results
Disclaimers: Dr Rubin is a member of the Novartis Speakers Bureau and has served on advisory boards for Novartis. Dr Corless has also served on advisory boards for Novartis.


